Day :
- Nanomedicines and Biomedical Applications | Pharmaceutical Nanotechnology | Nanomedicine and Drug Delivery | Nanotechnology for Targeted Drug Delivery | Regulatory Guidance for Pharmaceutical Nanotechnology | Recent Advances in Nanotechnology | Major Challenges in Nanotechnology
Location: Holiday Inn Rome - Aurelia Via Aurelia Km 8,400, 00163 Roma, Italy

Chair
Katharina M Fromm
University of Fribourg, Switzerland

Co-Chair
Helen McCarthy
Session Introduction
Katharina M. Fromm
University of Fribourg, Switzerland
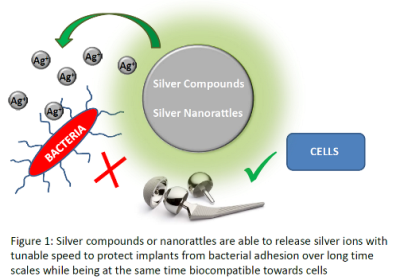
Title: Silver compounds and nanorattles: triggered drug delivery and prevention of implant infections
Time : 11:20-11:45

Biography:
Katharina M Fromm is a Coordination Chemist with expertise in the bioinorganic chemistry of silver, nanoparticles and nanocontainers as well as battery nanomaterials. She has been particularly interested in providing protective coatings for metallic and polymer implant materials and collaborated with microbiologists, infectiologists and medical doctors as well as industrial partners. Her concern is not only about antimicrobial activity, but also biocompatibility of nanomaterials and the bacterial resistance mechanisms towards metal ions such as silver.
Abstract:
Statement of the Problem: More and more artificial materials are implanted into the human body to replace bone and organ function in case of failure. These materials do not possess a natural defence system and are hence prone to bacterial adhesion which can occur during operation or via hematogenous seeding. Bacterial infections of implants may have severe consequences as they cannot be treated with traditional antibiotics.
Methodology & Theoretical Orientation: We developed coordination compounds based on ionic silver and a ligand to be attached on metallic implant surfaces. In parallel, silver nanoparticle containing nanorattles based on inorganic shell materials such as silica or titania were developed for a slow silver ion release. The compounds were thoroughly characterized in bulk as well as in the form of surface coatings. The antimicrobial activities were evaluated by in vitro Kirby Bauer tests as well as in vivo tests. Biocompatibility tests were performed on fibroblast cells in vivo. Both results were related to the silver ion release profiles from the materials in biological buffer media. A bacterial sensor has been developed to link the drug release from nanorattles to the presence of bacteria.
Findings: We have found several new coordination compounds based on silver ions that can be successfully attached to metallic implant surfaces to release antimicrobial metal ions while remaining biocompatible. For a prolonged release, we developed nanocontainers that provide drug protection and tunable release times and that can also be attached to implant surfaces. In order to target the release in a more controlled way, we developed a bacterial sensor able to recognize DNA material in a selective, fast and sensitive way for a specific treatment.
Conclusion & Significance: With the panoply of antimicrobial compounds, implant materials can be protected immediately after operation to prevent infections occurring during this process, as well as long-term protected against infections occurring via hematogenous seeding. The bacterial sensor allows to trigger drug release and can be used as analytical tool as well for rapid and specific diagnostics
Helen O McCarthy
Queen’s University Belfast, UK
Title: The RALA delivery platform: Altering the biodistribution of nucleic acids in vivo
Time : 11:45-12:10

Biography:
Helen O McCarthy has her research interest in the development of non-viral delivery systems for nanomedicine applications from last 11 years. These biomimetic systems are designed to overcome the extra and intracellular barriers, so that the macromolecular payload can be delivered at the destination site in order to exert the optimal therapeutic effect. She has designed and patented two delivery systems. Her research team involved in the development of a number of 2nd and 3rd generation multifunctional delivery systems
Abstract:
RALA is a 30mer cationic amphipathic peptide that condenses nucleic acid cargo into cationic nanoparticles (~50 nm diameter) suitable for gene delivery. However, upon systemic administration of plasmid luciferase-loaded (pLuc) RALA nanoparticles, bioluminescence is largely confined to highly vascularised organs, such as the lungs and liver. This represents a potential limitation of unfunctionalised RALA nanoparticles, which may not reach the tissues requiring the therapeutic cargo. The aim of this project is to functionalise RALA to increase circulation time and improve the pharmacokinetic profile of RALA nanoparticles. Vitamin E tocopherol polyethylene glycol succinate (TPGS) is a regulatory-approved non-ionic surfactant used in various drug delivery systems to achieve improved stability. TPGS was conjugated with five arginine residues (R5) to form TPGS-R5. Composite RALA/TPGS-R5 nanoparticles were complexed with plasmid DNA (pDNA) at a range of W:W ratios. Characteristics of nanoparticles formed were assessed by encapsulation assay, size and charge analysis. In vitro functionality was assessed by transfection studies in MDA-MB-231 breast and PC-3 prostate cancer cells. Stability studies analysing integrity of nanoparticles in serum and at physiological salt concentrations followed. In vivo biodistribution studies were performed in BALB/c SCID mice with either PC-3 or MDA-MB-231 xenografts. RALA/TPGS-R5 nanoparticles (W:W ratios 10:4, 8:6 and 6:8) carrying pLuc (50 µg) were delivered via tail vein injection. Bioluminescence was measured using a Bruker in vivo Xtreme imaging system 48 h and 96 h post injection. RALA/TPGS-R5 formed nanoparticles with pEGFP-N1 (~150 nm diameter and ~20 mV zeta potential) and transfected MDA-MB-231 and PC-3 cells. W:W ratios 10:4, 8:6 and 6:8 were stable at physiological salt concentrations. Functionalisation of RALA nanoparticles with TPGS-R5 reduced the luciferase expression detected in the lungs, liver, kidney and spleen. Up to a 30-fold increase in luciferase expression was detected in PC-3 tumours 48 h after treatment with 6:8, relative to untreated control. In MDA-MB-231 xenografts, a 12-fold increase in luciferase expression in tumours was detected, which was significantly higher (P≤0.05) than that of the RALA treatment group. Addition of TPGS-R5 to RALA nanoparticles improves the in vivo pharmacokinetics for the delivery of nucleic acids by reducing accumulation in the highly vascularised organs. This indicates the ability of TPGS-R5 to avoid clearance and increase circulation time of RALA nanoparticles in circulation. The enhanced transgene expression in tumours in both prostate and breast cancer models highlights the potential of this composite delivery system for systemic gene delivery, and warrants progression to studies involving delivery of therapeutic nucleic acids for a third generation cancer therapy.
Kyle M Jandrasitz
Microfluidics International Corporation, USA
Title: Microfluidizer® high-shear technology for manufacturing pharmaceutical drug delivery nanoparticles: From development to production
Time : 12:10-12:35

Biography:
Abstract:
Nanotechnology has revolutionized the pharmaceutical industry, especially with the applications of using nanoparticles (NPs) as drug delivery systems (DDS). These NPs show a number of advantages comparing to conventional methods such as increased bioavailability through enhanced solubility, forming theranostic platforms by co-delivering diagnostic and therapeutic agents, overcome various transport barriers to reach to the targeting sites, and controlled release capabilities, etc. Examples of such systems include nanoemulsions, liposomes and polymer nanoparticles. Despite their great potentials, major manufacturing challenges exist such as precisely control particle size and size distribution, achieve repeatable and scalable results, comply with all cGMP requirements and product sterilizable, e.g., through terminal sterile filtration. Microfluidizer® technology is an advanced technology that satisfies all of the requirements by delivering superior uniform shear and energy dissipation rates through the utilization of fixed geometry interaction chamber and constant process pressure. The unique benefits are presented here with three case studies. The first case study compares making an oil-in-water nanoemulsion adjuvant with post-processing sterile filtration using both of the Microfluidizer and traditional high-pressure homogenization technology. Microfluidizer was able to produce nanoemulsions have much higher filterability due to smaller droplets and narrower distribution. The process also showed excellent energy efficiency. The second and third case studies demonstrate producing and scaling up of a liposomal antibiotic formulation and fabricating two different polymer particles, one solid and the other one with embedded nanoparticles, respectively. In summary, Microfluidizer® technology is very efficient, reliable, and well-suited for manufacturing drug delivery nanoparticles for the pharmaceutical industry from development to production

Cristina M Sabliov
Louisiana State University, USA
Title: The effect of nanoparticle properties, detection method, delivery route and animal model on PLGA nanoparticles biodistribution in mice and rats
Time : 12:35-13:00

Biography:
Cristina Sabliov, PhD is a Professor in the Biological and Agricultural Engineering Department at Louisiana State University and LSU Agricultural Center. She is leading an international renowned research program in the field of Nanotechnology, specifically focused on polymeric nanoparticles designed for delivery of bioactive components for improved food quality and human health
Abstract:
The presentation is focused on poly(lactic-co-glycolic) acid (PLGA) nanoparticle (NP) biodistribution in mice and rats, based on a literature review. The nanoparticle presence expressed in % dose particles/g tissue in the liver, kidney, spleen, lung, heart, and brain were compared based on particle size, animal model, method of delivery and nanoparticle tracking method. The liver showed the highest uptake of particles in mice, and the lung showed the highest uptake in rats, with minimum amounts of nanoparticles in heart and brain. The concentration of particles decreased to 0% dose/g over 24 hours after a single dose of IV administered particles. Orally delivered nanoparticles showed little to no uptake within the first 24 hours. Particles with physically entrapped indicators were detected at higher concentrations than covalently labeled nanoparticles. It was concluded that more research is needed on oral delivery of PLGA NPs as well as detection beyond 24 hours to better understand fate of polymeric nanoparticles in-vivo required successful application of nanoparticles in drug delivery.
Christine Charrueau
Université Paris Descartes, France
Title: Self-emulsifying drug delivery systems for increased availability of resveratrol and enhanced anti-oxidant activity
Time : 14:00-14:25

Biography:
Christine Charrueau has completed her PhD from Paris-Sud University in France, and Postdoctoral studies from North Carolina University, Chapel Hill, USA. She is currently an Assistant Professor in the Laboratory of Pharmaceutics, Faculty of Pharmacy, University Paris Descartes, France, and she is a member of the UTCBS Research Unit (Unité de Technologies Chimiques et Biologiques pour la Santé - UMR CNRS 8258 -- Inserm U 1022). She has published more than 30 papers in reputed journals
Abstract:
Resveratrol (3,5,4’-trihydroxystilbene) has attracted considerable interest for its beneficial potentials for human health. However, its in vivo biological effects appear strongly limited by its low bioavailability. To overcome this problem, current strategies are turned towards the design of nano-sized formulations like nanoparticles, liposomes or self-emulsifying drug delivery systems (SEDDS), potentially allowing for an increase in its efficacy in human health by enhancing its stability, solubility or capacity to cross cellular membrane. In this context, we have developed SEDDS consisting in ternary combinations of oils, surfactants, and co-surfactants, and able to form nanoemulsions upon aqueous dispersion. Tested on bovine aortic endothelial cells, these SEDDS were able to significantly increase the membrane and intracellular concentrations of resveratrol. In addition, resveratrol nanoemulsions significantly improved the cell protection from H2O2-induced injury in comparison with a resveratrol ethanol solution. In the same way, in a human immortalized chondrocytic cell line (T/C28a2), resveratrol SEDDS were able to increase cellular tolerance towards resveratrol, to increase resveratrol cellular uptake, and to improve protection against oxidative stress-mediated death. Finally, the capability of SEDDS to enhance resveratrol permeation across rat intestine was tested on Ussing chambers. The absorptive fluxes of resveratrol from the nanoemulsions were significantly increased compared to an ethanol solution. Simultaneously, the presystemic metabolization pattern was modified, suggesting that SEDDS could modulate this important limiting factor to resveratrol systemic absorption. In conclusion, nanoemulsions prepared from SEDDS dispersion could be promising formulations for enhancing cellular uptake and oral delivery of resveratrol
Seitaro Kamiya
Nagasaki International University, Japan
Title: A physicochemical study on the preservation of nanoparticles
Time : 14:25-14:50

Biography:
Seitaro Kamiya has his expertise in evaluation and passion in improving the pharmaceutics and pharmaceutical technology. He focuses on increasing the efficiency of powder solidification of the nanoparticles and demonstrating the association between nanoparticles and saccharides. In addition, his chief concern is to elucidate the mechanism of association between nanoparticles and carriers
Abstract:
The importance of nanoparticle formulation is increasingly recognized in supporting pharmaceuticals development. Thus, maintaining a constant state in nanoparticles is an important major issue. A method involving lyophilization with the addition of saccharides can be used to maintain the state of nanoparticles. In drugs; however, this method has not been sufficiently discussed. In this study, trisaccharides, tetrasaccharides, and pentasaccharides were added to the nanoparticle suspensions, followed by rehydration of the samples, which had been either dried normally or freeze-dried. The particle diameter size after rehydration at that time was then measured. In addition, each saccharide was measured using a powder X-ray diffractometer and differential scanning calorimetry (DSC) device. We studied the association between the nanoparticles aggregation and the crystal form of saccharides and their mechanisms by using the obtained results of the data of particle size, powder X-ray pattern, and DSC curves. The diameter of the nanoparticles was maintained when it was freeze-dried, while particle aggregation occurred when normal dried samples were used. In addition, crystallinity crystalline saccharide was not observed in the freeze-dried group but was in the normal dried group.
Gerardo Byk
Bar Ilan University, Israel
Title: New biocompatible nanoparticles: Multistep chemical modifications and biological applications
Time : 14:50-15:15

Biography:
Gerardo Byk received his PhD (summa cum laude) at the Hebrew University of Jerusalem. In his PhD work, he developed a new generation of peptidomimetic molecules by the introduction of the new concept of backbone cyclization. Since August 1992, he has been in AVENTIS, where he was involved in the development of novel non-viral gene delivery complexes for gene therapy. He joined Bar Ilan University/Israel in 1999, was promoted to Associate Professor in 2002, where he is currently associated with the Marcus Center of Pharmaceutical Chemistry. His main scientific interests: peptide, peptidomimetics, combinatorial chemistry and gene therapy. Lastly, his group entered the field of nanotechnology with the design and development of biocompatible nanoparticles suited both for peptide synthesis, and for in vivo applications.
Abstract:
We have developed new biocompatible, non-degradable NPs well tolerated both in vitro and in vivo with the particularity that peptide synthesis can be carried out on their surface. Although the NP’s have a large range of well-defined sizes going from 20 to 400 nm, they are all composed of the same monomers. Their shell composition, in contact with the biological media, is uniformly composed of polyethylene-glycol, thus their biocompatibility remains high along the different sizes. A proposed peculiar mechanism of formation allowed maintaining uniform their shell composition. The conjugation of molecules to the NPs was a real challenge since they are nano-hydrogels with high colloidal stability that can only be dialyzed for eventual removal of reagents. Therefore we have designed and proved a novel solid phase peptide synthesis method for Merrifield synthesis on nanoparticles based on the embedment of the NPs in a permeable and removable magnetic matrix. The platform composed of the NPs and the synthetic peptide is a useful tool for imaging methods for intracellular localization of the NPs using microscopy as we have shown in vitro for PC-3 cells a system using TAT, NLS and TAT-NLS peptides on the nanoparticles, and for in vivo tracking using the Zebra fish model.
Sophia Hatziantoniou
University of Patras, Greece
Title: Nanolipidic carriers containing curcumin: Preparation and physicochemical characterization
Time : 15:15-15:40

Biography:
Sophia Hatziantoniou is an Assistant Professor at the Department of Pharmacy of the Patras University. Her research interests are focused on: Incorporation of drug molecules in nanosystems (liposomes, nanoemulsions, solid state lipid nanoparticles (SLN), polymeric systems, dendrimers), to improve the pharmacokinetic properties, bioavailability and pharmacological response in target tissues (tumors, lung, skin). Her research also focuses on the formulation of novel carriers of bioactive molecules into final products and study their characteristics (size distribution, zeta-potential, particle surface morphology, content of actives and excipients, active bioavailability, stability); Study of the interaction of bioactive molecules with model lipid membranes mainly by thermal analysis in order to design new formulations, as well as to predict their interaction with biological membranes; Development of cosmetics and topical pharmaceutical product. Safety assessment and evaluation of their efficacy using non-invasive biomechanical methods for claim substantiation.
Abstract:
Statement of the Problem: Nanoemulsions (NE), solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) are colloidal carriers for bioactive compounds. They are applied in therapeutic, diagnostic and cosmetic formulations. Curcumin (cur) is a polyphenol found in the rhizomes of the plant Curcuma longa L., and is traditionally used in the treatment of many diseases because of its multiple properties. The high lipophilicity of the molecule renders it difficult to incorporate in an acceptable final formulation. The above drawback as well as the intense color and reduced chemical stability of the molecule in light and air is attempted to be overcome by the use of nanotechnology. The aim of this work is to investigate the possibility of using the benefits of nanotechnology in the efficient topical delivery of curcumin formulated as nanoemulsions, solid lipid nanoparticles and nanostructured lipid carriers.
Materials & Methods: Three types of nanocarriers containing curcumin (NE-cur, SLN-cur & NLC-cur) and their corresponding control samples (NE-control, SLN-control & NLC-control) were prepared using triglycerides (Solid TG or Liquid TG or Solid-Liquid TG combination) and phosphatydylcholine (Egg PC or Soy PC) was also used as lipid phase. The particle size and their colloidal stability over time was assessed by Dynamic Light Scattering (DLS), after centrifugation or storage at 4oC. The incorporation efficiency of curcumin in different nanocarriers was determined by size exclusion chromatography (SEC) and UV-Vis spectroscopy. Their film forming capacity was examined by scanning electron microscopy.
Findings: NEs, SLNs and NLCs of high curcumin content were successfully prepared and physicochemicaly characterized. Their stability was monitored over a period of 90 days. The high percentage of the incorporated curcumin and the uniformity of the particle distribution as well as the retaining of these characteristics overtime are factors indicating that the nanostructured lipid carriers and solid lipid nanoparticles are more suitable carriers for curcumin in comparison to lipid nanoemulsions.
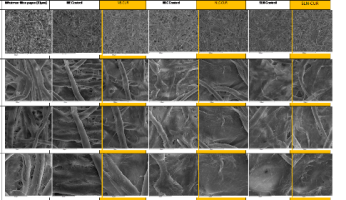
Figure 1: Film forming capacity of different nanocarriers as monitored by Scanning Electron Micoscopy
Tista Bagchi
University of Delhi, India
Title: Why ethical safeguards matter in medical nanotechnology? Prospects and problems in the delivery of nanomedicine via microsurgical interventions
Time : 15:40-16:05

Biography:
Tista Bagchi received her PhD from the University of Chicago in 1993 and an advanced diploma in Computer Science certified by the University of Oxford in 1999. She is currently a Professor in Linguistics at the University of Delhi and a Former Member of the Indian Council of Philosophical Research, besides being a current international member of the American Philosophical Association. She has also been a member of an inter-university Complexity Theory group and CSIR Mobility Scientist at the National Institute of Science, Technology, and Development Studies, Delhi, and has published several articles in Bioethics (alongside Linguistics and Philosophy).
Abstract:
Nanomedicine and medical nanotechnology are considered to constitute new frontiers of medicine on a number of counts. As with any such new developments in medical science and technologies, significant moral and prudential concerns, which together come under ethical concerns overall, inevitably arise as regards the regulation of the implementation and use of these developments. These concerns have been articulated with a good deal of clarity by Resnik & Tinkle (2007), who state that, among other things, “researchers, consumer advocates, and politicians have urged government agencies and private companies to proactively address the ethical, social and regulatory aspects of nanotechnology”. This need for ethical safeguards and regulations in nanomedicine gets compounded with the need for safeguards in microsurgical interventions given the prospect of combining the latter with, e.g., polymeric micelle-borne drug delivery. The mode of addressing the need for combined ethical safeguards and regulations adopted here is that of initiating reflective equilibrium with consideration of the respective prospects and problems of nanomedicine (including nanotechnological implementation) and microsurgery as known to date, with examination of certain key transnational guidelines in medical ethics, but focusing on this particular combination of the two with their considerable magnitudinal differences. While the prospects of combining protocols in nanomedicine and medical nanotechnology with those in microsurgery look highly promising, the potential problems and pitfalls are far better anticipated in advance for the formulation of reasonable ethical safeguards that benefit all.
Sabrina Gioria
JRC- European Commission, Directorate F – Health, Consumers and Reference Materials, Italy
Title: Nano medical products: Challenges, considerations and strategies
Time : 16:20-16:45

Biography:
Sabrina Gioria is based in the Directorate F-Health, Consumers and Reference Materials, at the Joint Research Centre (JRC) in Italy. His research interest lies in investigating the in vitro toxicity of engineered nanomaterials (NMs) covering the development and optimization of test methods, the study of nanoparticles (NPs) uptake including the route of internalization, as well as investigating the mechanisms involved in NPs toxicity. She is part of the CORE expert team of the European Nanomedicine Characterization Laboratories (EU-NCL) and she is involved in the JRC-Open-Lab activities
Abstract:
The rapid progress in the field of medical products involving nanotechnology makes the development of a coherent approach for their regulation a challenge. Robust and validated measurement methods are needed to allow informed regulatory decisions on their safety. Identifying critical physicochemical parameter that can have an impact on the products' quality and safety is extremely important. In particular, stability, size (-distribution), free vs. encapsulated drug content were considered as key parameters contributing to the biodistribution, pharmacokinetics (PK) and toxicity of nanoformulated drugs. Other parameters with an influence on the biological system are surface charge, surface chemistry, solubility and partition properties. Case studies of advanced methods for size-distribution, drug loading and drug-release in order to provide robust methods applicable to nanoproducts will be provided. In vitro toxicity testing is also an integral part for the identification of the hazardous potential of nano medical products in the early phase of their development. In vitro methods can provide initial insights of the toxic potential of e.g. a drug candidate at low costs and in a short timeframe. However, a caveat for the application of existing and highly standardized in vitro test methods for nanomedicine assessment is the interference of test reagents with the nanomaterial which can lead to false predictions of the assay. Here we address the pitfalls of existing toxicity testing when applied to nanomaterials and suggest alternatives. Progress in method development is essential to foster necessary standards that will support a harmonised regulation of nanomaterial-containing products. JRC scientific knowledge, technical competences and advanced instrumentation in the characterization of nanomaterials will be soon accessible to academia, public and industrial laboratories based within EU Member States and H2020 associated countries through the JRC Open Nano-Lab activities.
Ismaeil Haririan
Tehran University of Medical Sciences, Iran
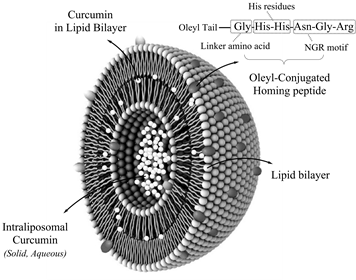
Title: Curcumin-loaded nanoparticles used for breast cancer treatment
Time : 16:45-17:10

Biography:
Ismaeil Haririan received the PharmD in Pharmacy by working on SAR (Structure-Activity Relationship) of drug molecules from State University of Tabriz (Iran) in 1986. He got his PhD in Pharmaceutics and Physical Pharmacy (1989-1994) from London School of Pharmacy (UK). Apart from some significant works on novel drug delivery systems and physic-mechanical studies on some pharmaceutical polymeric films, he turned his attention to biomaterials and nanotechnology. He co-operated with some other Tehran University Academic Staff to establish Biomaterials Research Center (BRC) in 2007. This allowed him to enter the new field of investigation of cancer gene therapy and drug targeting by applying pharmaceutical biodegradable polymer/non-polymer vectors. He is the Founder of Pharmaceutical Biomaterials as a new PhD field and Director of the Center for Research in Medical Biomaterials Research Center (MBRC) as well as the Director of Department of Pharmaceutical Biomaterials at TUMS.
Abstract:
To contrive against the obstacles in physicochemical properties of curcumin, various carriers and delivery systems have been introduced in the past two decades. Among a multitude of potential vehicles, the prospective effects of the liposomes and lipid-structures in improving the pharmacokinetic and dynamic of turmeric compounds have numerously been reported in recent studies. Besides, peptides that modulate cancer cell specific molecular pathways have a great potential as anticancer therapeutics. Among them, peptides that induce cell death via apoptosis have attracted ever-increasing attention. Tumor homing peptides and peptidomimetics containing RGD or NGR motifs have been exploited for targeting of therapeutics or diagnostics to cells with an overexpression of αυβ integrin family of adhesion receptors. On the other hands, studies have demonstrated that KLA peptides with a sequence of (KLAKLAK)2 can induce apoptosis in cancer cells. However, cell internalization is perceived as a major obstacle for development of such pharmaceutically useful peptides. Many efforts have been done to optimize ACP properties through two approaches: computational design and delivery systems. Among the carriers, gold nanoparticles offer a safe delivery platforms for anticancer agent development. Self-assembled structures were prepared from the oleyl-peptide at pH 3, 5.5, and 7. Curcumin was also dispersed in aqueous phase at neutral pH and was further separated from the colloidal particles and precipitates through filtration. According to the results, the more cytotoxicity and cellular uptake by T47D and MCF-7 breast cancer cells were observed for the smaller NPs in size an aspect ratio (AR) in which T47D cells was more sensitive than MCF-7 cells. The MTT results were confirmed by the morphological changes for the cancer cells exposed to NPs. Our finding suggested that the biological and pro-apoptotic effects of the mitochondrial targeting peptide were tuned by P-AuNPs upon their size and shape.
Sandra Carvalho
University of Minho, Portugal
Title: Surface modification: A promising solution for biomedical applications
Time : 17:10-17:35

Biography:
Sandra Carvalho studied Chemistry and Physics at University of Minho, Portugal and at same university, in 2004, she graduated as PhD in Physics (thesis work carried out in Portugal, France, Netherlands and Germany, in the field of hard PVD coatings). She is Head of the Research Surface Modification and Functionalization Group of the Centre Physics of University of Minho. She is involved in innovative nanoscale coating architectures for functional and smart surfaces such as flexible devices and bio-sensors, nanostructured coatings, nanostructured materials with barrier and antimicrobial properties, nanoparticles and 3D nanostructures. She is Head of the Laboratory of Corrosion and Electrochemical Studies. She is the Scientific Coordinator of Doctoral Program on Surface and Protection Engineering. She is member of European Joint Committee on Plasma and Ion Surface Engineering. She belongs to the Executive Committee of the Portuguese Materials Society.
Abstract:
Significant advances have been made in the technology of biomedical coatings and materials. This talk will provide an extensive review of coating types and surface modifications for biomedical applications. In many cases, side by side with traditional properties, like wear and corrosion resistance, biomaterials have to be compatible with body tissues and fluids, have anti-microbial activity and, in specific cases, contributing for a good integration of the material in the human body (e.g. osseointegration). It is difficult to find a unique material with all these functionalities, reinforcing the importance of surface engineering to supply new functionalities. The topic of this talk is centred in the surface modification of materials, traditionally used for biomedical applications, in order to improve their performance for specific cases. Different surface modification methods will be presented and the potential applications like orthopaedic (Figure 1), cardiovascular and urethral stents (Figure 2), biosensors (Figure 3), dental implants (Figure 4) will be discussed. Several cases studies from antimicrobial coatings to bioactive surfaces will be presented.
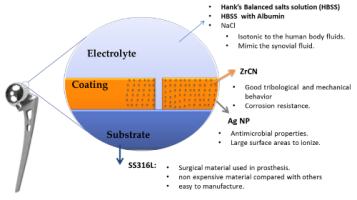

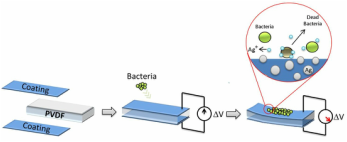
Figure 1: Functional coating for hip joint
Figure 2: Functionalized stents with antimicrobial activity
Figure 3: Smart electrode deposited on polymers based sensors for smart implants
Figure 4: New bioactive surface for dental implants
Francesca Ungaro
University of Napoli Federico II, Italy
Title: Overcoming biological barriers in severe lung diseases through tailored inhalable nanoparticles
Time : 17:35-18:00

Biography:
Francesca Ungaro is Associate Professor of Pharmaceutical Technology at the Dept. of Pharmacy of University of Napoli Federico II. Since her PhD, she has been studying innovative delivery systems for small and biotech drugs, with particular regard to micro- and nano-particles. In the last 10 years, special attention has been focused on engineered carriers for inhalation. In particular, she has been coordinating several national and international projects (1 ongoing) aimed to the development of inhalable formulations for cystic fibrosis treatment. She is author of 63 scientific articles in highly-ranked journals, 1 patent, 4 book chapters, 1 editorial and more than 100 presentations at symposia (h-index =22, total citations >1500).
Abstract:
Lung delivery represents a fascinating option to limit ubiquitous distribution of systemically, and often chronically, administered drugs used to treat severe pulmonary diseases. Nonetheless, clinical outcomes of inhaled therapies strongly depend on drug ability to deposit along the airways and to overcome barriers imposed by the lungs. In this context, the general aim of our studies is the development of inhalable nanomedicines able to deliver the intact drug in the lungs and to shield its interactions with lung lining fluids while enhancing drug availability at the cell target. This objective has been pursued through the design and production of differently engineered nanoparticulate systems with increasing levels of complexity, driven by technological and biological design rules. Some examples, such as drug nanocrystals, micelles and biodegradable poly(lactide-co-glycolide) (PLGA) nanoparticles, will be discussed highlighting how the most appropriate formulation approach can be selected only taking into account the distinct physico-chemical profile of the drug under investigation (e.g., molecular weight, solubility, stability) and the peculiarities of the lung pathology (e.g., cystic fibrosis, lung cancer). Surface engineering of nanocarriers with either polymers or phospholipids turns out as crucial to face the current challenge of overcoming lung barriers, especially mucus. Last but not least, in vitro/in vivo studies represent a critical step to select the best formulation to candidate for further development.
- Nanomedicines and Biomedical Applications | Pharmaceutical Nanotechnology | Nanomedicine and Drug Delivery | Novel Drug Delivery Technology | Nanotechnology for Targeted Drug Delivery | Nano Pharmaceuticals
Location: OLIMPICA 4

Chair
Jonghwi Lee
Chung-Ang University, South Korea

Co-Chair
Konstantinos Avgoustakis
University of Patras, Greece
Session Introduction
Jonghwi Lee
Chung-Ang University, South Korea
Title: Bio-inspired, microchanneled materials prepared by crystallization of solvents
Time : 10:45-11:10

Biography:
Jonghwi Lee obtained his PhD degree in University of Michigan, Ann Arbor and worked for Merck Research Laboratories as a Senior Researcher after his Postdoctoral training at the University of Minnesota. He has won prizes from The Polymer Society of Korea (Best Paper Award), Korean Society of Industrial Engineering Chemistry (Contribution Recognition Award, Best Paper Award, Best Industry Collaboration Award), and Chung-Ang University (Excellence in Achievement Award, Bae Young Soo Award). He has published more than 150 research papers, and currently a Vice Editor of Journal of Industrial and Engineering Chemistry (IF = 4.179) and Macromolecular Research.
Abstract:
Microporous polymeric materials in nature have well-controlled structures of skeletal walls and pores for their functions. The structures of man-made microporous polymers are commonly limited to isolated porous architecture, although they have continually been developed for the critical roles in various industries. The directional melt crystallization of solvent, a relatively new versatile preparation method to produce aligned pores in the forms of 3D patterns, has produced porous structures of Voronoi and honeycomb-like architecture morphology. By applying this technique to polymers, we have produced various materials having ordered microchannels. Crystallization rate and direction have been carefully controlled in a home-made apparatus to prepare defect-free materials having well-ordered through-thickness microchannels. From polymer solutions or dispersions, solutes become skeletal portion and crystallized solvents become pores after sublimation. The free-standing membranes of 60-90 vol% through-thickness porosity could be prepared without having internal microcracks. With the support of nanotemplates, nanospheres, nanorods, and nanomembranes could be prepared too. Controlling pore morphology by directional freezing offers a versatile route to prepare unique porous polymer and composites for future biomedical, electronics and environmental applications.
Konstantinos Avgoustakis
University of Patras, Greece
Title: Multifunctional magnetic nanocarriers of anticancer drugs
Time : 11:10-11:35

Biography:
Konstantinos Avgoustakis obtained his Diploma in Pharmacy from Aristotle University of Thessaloniki, Greece in 1985 and a PhD degree in Pharmaceutical Technology from King’s College, University of London, UK. Since 1994, he has joined the Department of Pharmacy in University of Patras (Greece), where he teaches subjects related to Pharmaceutics and Drug Delivery and since 2015 he has joined Biomedical Research Foundation of Athens Academy as Collaborating Researcher. His research interests lie on the controlled, targeted therapeutics delivery using engineered nanoparticles. He is the author of 60 articles in peer-reviewed journals and 1 article in Biomaterials Encyclopedia. He is also the author (inventor) of 1 European patent. His published research has received over 2000 citations with an h-index of 23. He is an Assistant Editor of the scientific journal Current Nanoscience. He has participated in several research programs in collaboration with academic and industrial organizations (in 7 as coordinator).
Abstract:
Magnetic nanoparticles have been intensively investigated for the selective delivery of anticancer agents. Magnetic drug nanocarriers provide further advantages as they concomitantly allow for tissue imaging through magnetic resonance imaging and can induce cell-destructive hyperthermia in tumor by application of external alternating magnetic fields. We have been investigated the application of hybrid organic/inorganic magnetic nanoparticles for a more selective delivery of potent anticancer agents to tumors. In this context, we developed recently pH-responsive magnetic nanocarriers based on the graft-copolymer of poly (methacrylic acid)-g-poly(ethyleneglycol methacrylate) for the controlled delivery of cisplatin. Enhanced in vivo anticancer therapeutic efficacy and reduced toxicity was recorded for these cisplatin nanocarriers in comparison to the free drug cisplatin, particularly when a magnetic field gradient was applied at the tumor site. A problem frequently encountered with drug nanocarriers is their low level of uptake by tumor cells. In order to overcome this problem, we have developed poly(lactide)-poly(ethyleneglycol) magnetic nanocapsules functionalized with a cell penetrating TAT peptide and loaded them with paclitaxel. The conjugation of the TAT peptide on the surface of the nanocapsules resulted to highly increased uptake of nanocapsules by A549 cancer cells and to a profound improvement of the cytotoxicity of the paclitaxel-loaded nanocapsules against the cancer cells. In order to increase magnetic responsiveness, we developed colloidal clusters of iron oxide nanocrystals (MIONs), particularly in the condensed pattern (co-CNCs). These MION-based co-CNCs were characterized by high magnetization and high relaxivities combined with remarkable colloidal stability and drug-loading properties. Hypoxia develops in solid tumors, which leads to the development of resistance of tumor cells to radiotherapy and chemotherapy. We are working on magnetic nanocarriers for the targeted (selective) delivery of sodium-glucose transporter protein inhibitors to tumors. This nanomedicine combined with radiotherapy or chemotherapy would represent an effective treatment of hypoxic tumors.
Khaled Greish
Arabian Gulf University, Bahrain
Title: The use of Styrene Maleic Acid (SMA) nanomicelles for effective oral delivery of bioactive therapeutics
Time : 11:35-12:00

Biography:
Khaled Greish graduated from the Faculty of Medicine, Suez Canal University, Egypt in 1992. He received his Master’s degree in Clinical Oncology in 1997. From 1999 to early 2008, he joined the lab of Professor Hiroshi Maeda, a world leader in the field of Anticancer Nanomedicine. Along with Professor Maeda, he developed an affordable platform nanotechnology micellar system for targeting anticancer drugs to solid tumors. In 2008, he moved to The University of Utah, as Research Assistant Professor. He joined Otago University in 2011, and currently Associate Professor at Arabian Gulf University, Bahrain. His areas of interest span the formulation and characterization of different advanced drug delivery systems, anticancer drug discovery/development, tumor vascular biology and animal tumor models. He was awarded the CRS Postdoctoral Achievement award in 2008 and, in 2010 he was elected as member of the CRS College of Fellows
Abstract:
The oral route is the most preferred mode of administration among patients, due to its non-invasive nature. Oral administration of therapeutics can improve the patient`s quality of life, reduce costs associated with hospitalization and avoid complications associated with intravenous injections. The primary barrier to clinical application of an oral therapeutic, is poor oral bioavailability, a result of the combination of low water solubility, low pH stability, poor mucosal penetration, extensive first pass metabolism, and P-glycoprotein (P-gp) efflux and enzymatic degradation. Nanocarriers present a unique opportunity to overcome these barriers due to their design flexibility, surface functionality, and ability to deliver a wide range of therapeutics. Nanocarrier systems can enhance the solubility of the bioactive, protect them from enzymatic degradation and allow incorporation of the appropriate surface functionalization to favor uptake through the intestinal epithelium. Additionally, nanocarriers can prevent the direct contact of the bioactive agent with the cells of gastrointestinal (GI) tract. Nanocarriers can thus reduce the toxicity, prolong the circulatory half-life of the bioactives and accommodate targeting moieties to enhance further their prospects as site specific delivery systems. In this presentation, we will discuss our work utilizing Styrene Maleic Acid (SMA) nanomicelles for oral drug delivery through two case studies. The first example involves the use of oral paclitaxel nanomiceller formulation for treatment of colon cancer. The second case will discuss the successful use of oral nanoformulation of the selective estrogen receptor modulator (raloxifene) for treatment of inflammatory bowel disease (IBD).
Katja Bettina Ferenz
University of Duisburg-Essen, Germany
Title: Perfluorodecalin-filled albumin nanocapsules as artificial oxygen carriers
Time : 12:00-12:25

Biography:
Katja Bettina Ferenz has completed her PhD in Pharmaceutical Chemistry from the “Westfälische-Wilhelms-University Münster” in Germany. Since 2011, she leads her own research group, Development of Artificial Oxygen Carriers at the University of Duisburg-Essen, University Hospital Essen, Institute of Physiological Chemistry, Essen, Germany. She has published 14 papers in reputed journals and participates in the professional training of physicians specialized in transfusion-medicine with lectures on actual developments in the field of artificial oxygen carriers. Her research interests are artificial oxygen carriers, micro- and nanoparticles, nanomedicine, perfluorocarbons, drug delivery and biomaterials
Abstract:
Despite long lasting efforts, at present a harmless, effective artificial oxygen carrier is missing for clinical use both in Europe and USA. To bypass this, bottleneck albumin-derived perfluorocarbon-based nanocapsules (nanocapsules) were designed as a novel artificial oxygen carrier. Most importantly, nanocapsules do not contain any chemical emulsifier. Nanocapsules are synthesized in different size ranges (Ø 100-1500 nm) by using either ultrasonics or a microfluidizer apparatus. Physical assessment of size (DLS, REM/LSM), oxygen transport capacity or the charging of erythrocytes is performed. In different animal models of the rat (topload/ normovolemic hemodilution), physiological parameters (e.g. breathing, blood pressure), blood gases, electrolytes and signs of tissue impairment in plasma (e.g. ASAT, LDH) are monitored. Microcirculation of the liver (IVM) and intravascular half-life of nanocapsules and their multiple loading with O2 are measured (NMR). Successful in-vitro experiments concerning the oxygen transport capacity of the nanocapsules (oxygen supply of yeast cells, oxygenation of desoxy-Hb inside erythrocytes) and the proof of bio-functionality in the isolated organ (Langendorff-heart) were followed by in vivo experiments (rat) investigating toxicity and pharmacokinetic. Most interestingly, relevant changes in systemic parameters during and after i.v. infusion of nanocapsules were not detected. Microvascular perfusion and oxygen supply by erythrocytes remained unaffected. Parameters indicating tissue impairment did not show any life-threatening deviation. Intravascular half-life of nanocapsules was satisfactory. The subsequent “proof-of-concept” study (rat) to demonstrate the functionality in the complete animal was successful. All animals treated with nanocapsules survived the gradual exchange of about 95% of blood much better than control animals.
Barbara Palazzo
Ghimas S.p.A. c/o Dhitech Scarl, Campus Ecotekne, Italy
Title: Nanostructured ceramics for bone cancer therapy
Time : 12:25:12:50

Biography:
Barbara Palazzo has completed her PhD in Chemistry in 2006 from Bologna University and Postdoctoral Studies from Oriental Piedmont University, Medical Science Department. She is responsible for the local research unit of Ghimas S.p.A in the High Tech District of Lecce. She is author of more than 35 papers in peer review journals. Her research is focused on biomimetic inorganic and polymeric materials intended for the repair of defects in the maxilla-facial site or in the osteochondral unit. She investigates multifunctional nanomaterials for the controlled delivery of chemotherapeutic agents, above all for the treatment of bone tumors and metastasis.
Abstract:
Bone cancer, is among the most invasive tumors with lowest survival probability. In this case, following cancerous bone excision, concealing the bone tissue loss is necessary and to this aim materials designed for bone replacement are requested. Moreover, a subsequent antitumor treatment needs to be carried out to avoid recurrence. This review talk will focus, three particular strategies, all of them aimed to address the therapeutic treatment to the tumour site: 1. The employment of ceramic nanoparticles as targeted anticancer drug carriers to be exploited as injectable devices. Particularly the speech will include calcium phosphate and silica-based materials intended for hosting and carrying anticancer molecules, due to their particular surface nanostructure. 2. the use of those nanomaterials that we can define as “intrinsically anticancer” because they possess some properties that can activate specific cellular mechanism, and specifically, they can act as nano-thermoactuators for hyperthermia or as small sources of radiation. 3. the use of nanobioceramics as bone fillers with anticancer function to be implanted into the affected piece of bone. This talk section will illustrate the combination of both the above described approaches: magnetic phases are included into implantable bioceramics in order to obtain composites that can be designed for skeletal reinforce and contemporarily act in order to prevent metastases after tumour resection. Starting from nanostructured bioceramics composition and functional properties, the results presented in this talk will describe the mechanism through which they may be adjuvant in the multifaceted curative techniques that must be adopted against bone tumours.
- Young Reasearch Forum
Location: OLIMPICA 4
Session Introduction
Ruba Ismail
University of Szeged, Hungary
Title: Up to date advances in nano-carrier systems for oral delivery of antidiabetic peptides
Time : 14:10-14:25

Biography:
Ruba Ismail has completed her Master’s study in Pharmaceutical Sciences in 2015 from Tishreen University, Latakia, Syria. Presently, she is a 1st year PhD student at Szeged University, Faculty of Pharmacy, Institute of Pharmaceutical Technology and Regulatory Affairs, Szeged, Hungary.
Abstract:
Current progresses in pharmaceutical biotechnology have led to the discovery of numerous antidiabetic peptides. Nevertheless, these drugs like insulin, GLP-1 and its analogs are currently administrated parenterally, a route which is not well accepted by patients, and also does not mimic the endogenous pathway of insulin or GLP-1 secretion. Aiming to overcome the various disadvantages of this delivery route, tremendous efforts have been recently devoted for oral antidiabetic peptides administration. Based on the evaluation of recently published papers, it can be concluded that among numerous approaches to enhance the antidiabetic peptide oral bioavailability, much of the success was recorded using different nanocarrier systems including solid lipid nanoparticles, micelles, polymeric nanoparticles, liposomes and nanoemulsions. A successful nanocarrier should protect the peptide from the harsh environment within the GIT, and facilitate both mucus permeation and epithelial absorption. Besides, there is an integral need to prove that the benefits of these nanosystems overweight their risks, further in vivo and human studies must be conducted to know more about the effect of these carriers on the pharmacokinetics and efficacy of antidiabetic drugs. Despite these significant research efforts, there is still no FDA approved oral insulin or GLP-1 analog on the market. Thus, research in this area should be continued at even a higher pace to access a safe and effective nanosystem that could be completely accepted for chronic treatment of diabetes. This review emphasizes the challenges, advantages and limitations of different possibilities with a critical thinking and aiming to select the proper way for further steps in the field.
Annalisa Dalmoro
University of Salerno, Italy
Title: Ultrasonic energy as effective tool to improve the production methods of lipid and polymeric drug delivery systems
Time : 14:25-14:40

Biography:
Annalisa Dalmoro carries out her research work in TPP group at the University of Salerno on novel processes for the production of dosage forms and on the development of materials for biomedical applications. In particular, she focused her work on the design and building of a semi-continuous bench-scale apparatus for the production of shell-core microparticles combining two non-conventional technologies, such as ultrasonic atomization and microwave drying; on the production of micro and nano systems by exploiting ultrasonic energy; on the synthesis of enteric polymers for pharmaceutical applications; on the study about the crosslinking phenomena of biopolymers, thus about their applicability for in situ coating of coronary stents. Moreover, she was also involved in studies on granulation processes, stabilization of food by spray drying, and on fruits, grains and legumes processing by microwaves, through collaborative projects with companies.
Abstract:
Statement of the Problem: The large interest in the development of lipid and polymeric micro- and nano- drug delivery vectors lies in their properties of minimizing drug loss or degradation, reducing side effects, improving drug biodistribution and penetration in cellular compartment. Different advanced preparation techniques are currently used, however as all modern manufacturing processes also pharmaceutical industry must respond to the mandatory rules of sustainable productions. Therefore it is moving to the intensification of production processes, included those based on nanotechnologies, i.e. to process miniaturization, capital cost reduction, and improvement of energy use and final product quality. Among the tools to achieve this objective, ultrasonic energy has the potential for the improvement of production techniques of lipid and polymeric carriers on micro and nano scale.
Methodology & Theoretical Orientation: Ultrasonic atomization and sonication were the method used for the “intensified” production of drug delivery vectors, respectively. The fundamentals of both were analyzed in order to understand the mechanisms at the basis of micro- and nano- vectors formation and sizing.
Findings: The ultrasonic energy was a potent tool able to produce vectors encapsulating different active molecules, in particular to: 1) produce enteric shell-core microparticles by energy-saving ultrasonic atomization, 2) make stable double emulsions for the production of polymeric micro- and nanoparticles, 3) size the final dimension of liposomes, according to the application requirements.
Conclusion & Significance: Advantages and novelties introduced by the use of ultrasonic energy in preparative methods of lipid and polymeric vectors were put in evidence by proving that the ultrasonic-based techniques are versatile and promising for the successful preparation of stable, highly loaded formulations, avoiding any harsh preparative conditions or large amount of solvents.
Abdulrahman M Elbagory
University of the Western Cape, South Africa
Title: Green synthesis of gold nanoparticles from South African plant extracts for the treatment of skin infection wounds
Time : 14:40-14:55

Biography:
Abdulrahman M Elbagory holds a MSc in Organic Chemistry from the University of the Western Cape (UWC) in 2015 in which he was awarded the National Research Foundation (NRF) fellowship. His Master’s study was about the chemical isolation and characterization of natural products from marine animals. His research included in vitro antiproliferation activity of the isolated secondary metabolites against cancerous cell lines and investigating their apoptosis properties. Currently, he is in third year of his PhD in the Department of Biotechnology (UWC), which is funded by the NRF and DST/Mintek Nanotechnology Innovation Centre. His PhD study is about the green synthesis of metallic nanoparticles from plants and their anticancer and antibacterial evaluations. He has two publications as first author and one review as a co-author.
Abstract:
Statement of the Problem: The preparation of gold nanoparticles (AuNPs) involves a variety of chemical and physical methods. These methods use toxic and environmentally harmful chemicals and procedures. Consequently, the synthesis of AuNPs using green chemistry has been under investigation to develop eco-friendly and biocompatible nanoparticles using plant-derived phytochemicals. Several green synthesized AuNPs have been shown to have antibacterial effect. There is growing need for effective and safe antimicrobial agents to treat infected deep wounds. The aim of this study was to green synthesize AuNPs from plants, evaluate their antibacterial activity against skin wound infection bacteria including methicillin-resistant Staphylococcus aureus and also measure their toxicity on human normal fibroblast cell line (KMST-6).
Methodology: The AuNPs were biosynthesized according to Elbagory et al. (2016) from aqueous extracts of the South African Galenia africana and Hypoxis hemerocallidea plants. The AuNPs were characterized using Ultra Violet-Visible Spectroscopy, Dynamic Light Scattering and High Resolution Transmission Electron Microscopy. The antibacterial activity of the biosynthesized AuNPs were tested using Alamar blue assay. The toxicity of the biosynthesized AuNPs was evaluated in vitro using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay.
Findings: Spherical AuNPs were formulated ranging in size from 15 to 25 nm in diameter (Figure 1). The AuNPs from H. hemerocallidea were shown to have antibacterial activity against the tested bacteria strains except Salmonella sp., whereas Galenia-AuNPs only exhibited antibacterial activity against Pseudomonas aeruginosa (Table 1). Both AuNPs showed no toxicity on KMST-6 cells at the highest tested concentration (32 nM) after 24 hrs treatment.
Conclusion & Significance: Different AuNPs were successfully synthesized from plants using green nanotechnology. The results reveal that these AuNPs have antibacterial effects that can be safely employed in wound infections.
Nicole R S Sibuyi
University of Western Cape, South Africa
Title: Development of multifunctional gold nanoparticles for selective induction of apoptosis in target cells
Time : 14:55-15:10
Biography:
Nicole R S Sibuyi obtained her PhD degree in Biotechnology from University of the Western Cape (South Africa) where she is currently enrolled as a Postdoctoral Research Fellow under DST/Mintek Nanotechnology Innovation Centre (Biolabels Unit). Her research interests range from biomarker discovery, proteomics, targeted nano-based therapy and drug delivery. Her research is mainly focused in identifying biomarkers for chronic diseases such as cancer, diabetes and obesity. These disease-associated biomarkers will be used for the development of diagnostics as well as personalized therapy using Nanotechnology. Her research interests had advanced into Green Nanotechnology after she had received 3 months training in the application of green nanotechnology in cancer therapy at the Institute of Green Nanotechnology, Missouri University (Columbia, USA). They (Biolabels Unit) are currently using Green Nanotechnology for synthesis of biocompatible metallic nanoparticles using indigenous medicinal plants.
Abstract:
Development and progression of chronic diseases such as obesity and cancer, is dependent on angiogenesis for nutrients and oxygen supply to diseased cells. As such, pharmacological inhibition of angiogenesis is therefore a sensible strategy for treatment of these diseases. The aim of this study was to develop targeted anti-angiogenic gold nanoparticles (AuNPs) that can be delivered selectively to the target cells and trigger the apoptotic cell death. The AuNPs were bi-functionalized with a targeting peptide and a pro-apoptotic peptide. The targeting peptide (adipose homing peptide, AHP) used in the study binds to a protein that is overexpressed by endothelial cells in the white adipose tissue (WAT) vasculature of obese subjects. We previously evaluated the bio-distribution of nanomaterials functionalised with the AHP and demonstrated that these nanoparticles accumulated in the WAT of animal models of obesity. In the current study, the bi-functionalized AuNPs were synthesized then characterised by UV-Vis, Zeta potential and TEM. The selective targeting and toxicity of the targeted-AuNPs was investigated on three human cancer cell lines (Caco-2, MCF7 and HT29), of which Caco-2 cells express the cell surface receptor for AHP. The AuNP toxicity on cells was evaluated using the WST-1 and the APO Percentage assays, while the AuNP uptake was confirmed by ICP-OES analysis. The AuNP cytotoxicity was more pronounced in the cells expressing the receptor for AHP, the Caco-2 cells. The uptake of the bi-functionalized AuNPs was higher on target cells, the bifunctionalized AuNPs showed receptor mediated targeting and targeted destruction of Caco-2 cells following apoptosis pathway. The bi-functionalized AuNPs demonstrates potential for the development of targeted anti-angiogenic strategy for the treatment of obesity and possibly also colon cancer. The therapeutic efficacy and specificity of bi-functionalized AuNPs in animal models of obesity and cancer is underway.
Congcong Lin
Hong Kong Baptist University, Hong Kong
Title: Targeted drug delivery with dual-ligand decorated liposomes by anti-CA IX antibody and tumor homing cell penetrating peptide in 2D and 3D lung cancer cells
Time : 15:10-15:25

Biography:
Congcong Lin has her expertise in development of novel drug delivery system and passion in improving the health and wellbeing. Her main research direction is design and evaluation of the new drug delivery system based on the special microenvironment of tumor sites to achieve targeted drug delivery and precision therapy. She has developed triptolide-loaded liposomes decorated with anti-carbonic anhydrase IX (CA IX) antibody targeting CA IX confined to cancerous cells to increase the accumulation of the drug loaded liposomes at tumor sites and combining the advantage of pulmonary delivery for better retention of active ingredients in lung. This study provides insight into targeted and sustained delivery of a toxic drug through CA IX-decorated liposomes via the pulmonary route for lung cancer therapy.
Abstract:
Statement of the Problem: Lung cancer is one of the most common lethal malignancies worldwide with 1.59 million deaths each year. Low accumulation of therapeutic agents in the tumor site and lack of penetration ability of a drug delivery system into tumor masses are the main obstacles in efficient lung cancer therapy.
Aim: The purpose of this study is to enhance the efficacy of anti-cancer drug against lung cancer by targeting carbonic anhydrase IX (CA IX) confined to cancerous cells to increase the accumulation at tumor sites and combining the advantage of tumor homing cell penetrating peptide (CPP33) to promote the penetration of the drug loaded liposomes into lung cancer cells.
Methodology & Theoretical Orientation: Anti-CA IX antibody and CPP33 dual-ligand triptolide-loaded liposomes (dl-TPL-lip) were developed using the post-insertion technique. The characteristics of dl-TPL-lip including particle size, entrapment efficiency and drug release property were carried out. The effects of dl-TPL-lip on NSCLC cells were evaluated by wound healing and apoptosis assay. Moreover, the penetrating ability and inhibition efficacy of dl-TPL-lip were further investigated using 3D tumor spheroids.
Findings: The dl-TPL-lip displayed the optimal efficacy in inducing apoptotic feature of NSCLC cells, which showed tunable size (137.6±0.8 nm), high encapsulation efficiency (86.3±2.6) and sustained release. The superior penetrating ability and inhibitor effect on 3D tumor spheroids were also observed for dl-TPL-lip.
Conclusion & Significance: The data suggest that dual-ligand modification with anti-CA IX antibody and CPP33 on the surface of liposomes endow great potential for lung cancer therapy.
Xue Zhang
Hong Kong Baptist University, Hong Kong
Title: Cancer cell specific-penetrating peptide modified liposome for targeted delivery of anticancer agent to hepatocellular carcinoma
Time : 15:25-15:40

Biography:
Xue Zhang has her expertise in liposomal drug delivery systems in improving chemotherapeutic drug properties and for targeted cancer treatment. She has developed a cancer cell-specific liposomal carrier by modifying liposomal surface with a cancer cell-specific penetrating peptide. And also she evaluated the therapeutic efficacy and effectiveness of this carrier in vitro (2D and 3D cells) and in vivo hepatocellular carcinoma model.
Abstract:
Statement of the Problem: Nowadays liver cancer has become the second leading cause of cancer related death globally. Systemic therapy with chemotherapeutic agents has severe toxicity to normal cells and the application is limited in pre-clinical study. Researchers have reported that liposomal drug delivery system working as a carrier by encapsulating chemotherapeutic agents into its hydrophobic or hydrophilic parts can enhance the bioavailability and solubility of drugs, facilitate the tumor-specific targeting treatment purpose with surface modification, and further reduce the drug toxicity to normal tissues. However, these delivery systems have not been adopted in clinics. The purpose of this study is to develop cancer-cell specific penetrating peptide modified liposomal delivery system with CTD encapsulated for targeted hepatocellular carcinoma treatment.
Methodology & Results: The cancer cell-specific penetrating peptide (CCP) modified-liposome prepared in the present study had a particle size around 120 nm and a narrow polydispersity index. It had an enhanced cytotoxicity on HepG2 cells compared to the control, free drug and unmodified liposome by MTT assay. In addition, a provoked apoptosis was induced by this liposome as well. The cellular uptake results of HepG2 and normal Miha cells further confirmed the higher ability of the CCP-modified liposomes to penetrate cancer cells. A higher efficiency of delivery by CCP-modified liposomes as compared to unmodified liposomes was evident by evaluation of the HepG2 tumor 3D spheroids penetration and inhibition experiments as well as the in vivo study. In conclusion, our CCP-modified liposomes improve the anticancer potency of drugs for hepatocellular carcinoma.
Julia Ernst
Friedrich Schiller University Jena, Germany
Title: Polymeric micro- and nanocarriers for the treatment of biofilm-associated infections in cystic fibrosis
Time : 15:40-15:55

Biography:
Julia Ernst is a Pharmacist by training and graduated at the Friedrich Schiller University Jena in 2012. After gaining practical experience in pharmaceutical industry, hospital and public pharmacy in Switzerland and Germany, she started her PhD studies in 2014 at the Department of Pharmaceutical Technology, FSU Jena. Her research is focused on formulations for nanoparticular drug delivery systems to the lungs.
Abstract:
Antimicrobial treatment is a cornerstone of cystic fibrosis (CF) therapy, as approximately 90% of CF patients die from lung destruction promoted by pathogens such as Pseudomonas aeruginosa and Burkholderia cepacia. However, the efficacy of the inhaled antibiotics is limited due to a hindered penetration of drugs through mucus and bacterial biofilms which is difficult to imitate in vitro. In our experiments we developed a way to overcome these barriers, by loading tobramycin (Tb) into biodegradable poly(lactic-co glycolic acid) based nano- (NP) and microparticles (MP) modified with PEG and a blue fluorescent dye (AMCA) to investigate the penetration in mucus and the antibiotic efficacy in bacterial biofilms. Tb-loaded particles of 830 nm (MP) or 230 nm (NP) and zeta potentials of ca. -10 mV were prepared by a double-emulsion evaporation method and characterized by SEM and HPLC. For biofilm experiments, bacteria were cultivated in artificial mucus (AM)-containing chamber slides to allow the formation of a biofilm close to those of CF patients or in a microfluidic device to imitate the physiological shear flow in the body. The excellent penetration abilities of Tb-loaded particles through AM and biofilms and the remarkable antimicrobial efficacy in comparison to the free drug was confirmed by confocal laser scanning microscopy of LIVE/DEAD® stained biofilms. In conclusion, we demonstrated that the NP and MP displayed excellent properties as biocompatible, mucus-penetrating delivery systems for antibiotics with improved deposition and bacterial killing of biofilm-embedded pathogens even under more physiological conditions compared to conventional in vitro models.
Pegah Esmaeilzadeh
Martin Luther University of Halle-Wittenberg, Germany
Title: A responsive thiolated polysaccharide multilayer nanocoating for tuning cell adhesion and cell detatchment
Time : 16:10-16:25

Biography:
Pegah Esmaeilzadeh researches in the field of synthesis and characterization of single-walled natural protein nanotubes, and their byproduct architectures such as protein nanofibers, nanospheres, and nanoparticles have been successfully developed in nanotechnology section of RIPI of Tehran (Research Institute of Petroleum Industry). She was also active in different research projects on ZnO quantum dots as novel antibacterial, antifungal, or anticancer drugs. She is currently PhD student in Institute of Pharmacy in Martin Luther University Halle Wittenberg in Germany, and receiving new experiences in medical nanocoatings/interfaces/thin films and particularly cell studies.
Abstract:
Advanced efforts in the design of responsive biomedical coatings are focused on the decoration of surfaces with functionalities that promote a predetermined biological response such as modulating cell adhesion and proliferation as well as cell detachment. Following this aim, we synthesized a stimuli-responsible cell carrier nano-coating system with multilayers made of thiolated chitosan (t-Chi) and thiolated chondroitin sulfate (t-CS) units. This redox-responsive multilayer system was realized by intrinsic cross-linking triggered by oxidative stimuli, while these bridges were prone to dissociation under reductive conditions. It is remarkable that this chemical changes were fully reversible as demonstrated by repeated oxidation and reduction (Oxi-to-Re) or in opposite from reduction to oxidation (Re-to-Oxi) cycles. The physical properties of multilayers during these treatments were studied by in situ spectroscopic ellipsometry and liquid-based atomic force microscopy and surface plasmon resonance showing changes of layer thickness and elastic modulus. Since protein adsorption is a prerequisite of cell adhesion, binding of fibronectin was studied with a fluorescent labeling technique. Correspondingly, the novel multilayer nano-coating was used to define the human dermal fibroblast cell microenvironment and the impact of switching cycles on cell adhesion and detachment events was verified. The latter is presented briefly in figure 1. As this thiolated polymeric system is responsive to the body’s internal stimuli like pH and redox, it holds great promise for medical applications and stimuli-sensitive drug delivery systems.
Sophie Franceschi
Université Paul Sabatier, France
Title: Evaluation of organogel nanoparticles as new vehicle for lipophilic compounds
Time : 16:25-16:40

Biography:
Sophie Franceschi has her expertise in chemical and physicochemical studies of organized molecular systems for drug delivery and material applications. She has 20 years of experience in research and teaching. She graduated with a PhD from the Paul Sabatier University of Toulouse (France) in 1997 and has a Master’s degree in Molecular and Supramolecular Chemistry. Currently, she is Researcher in SMODD group in the IMRCP Laboratory at the University Paul Sabatier of Toulouse and also Teacher at Paul Sabatier University.
Abstract:
In recent years, a growing interest has emerged in the development of semi-solid colloidal careers for the delivery of water-insoluble drugs. Solid lipid Nanoparticles (SLN) and Nanostructured Lipid Careers (NLC) are example of such systems. As an alternative to SLN, we propose the use of an original family of organogel nanoparticles. Organogels are semi-solid materials in which an organic solvent (e.g., vegetable oil) is entrapped in the three-dimensional fibrous network formed by self-aggregation of a low molecular mass organic gelator (12-Hydroxystearic acid). The preparation process of the gelled oil nanoparticles is based on the sol-gel phase transition of the organogel obtained by hot emulsification (T°>T°gel) in presence of an aqueous solution of stabilizing agent (Polyvinylalcohol 80), leading to a stable semi-solid dispersion after cooling (T°
